Ozone Overview
Ozone is produced by lightning, the ultraviolet rays from the sun passing through the atmosphere, from UV radiation produced by special light bulbs or from high voltage corona discharge in an atmosphere containing oxygen. It is very unstable and quickly reverts back to oxygen. Ozone has a very distinctive odor and is easily recognized at levels far below the toxic range.
Ozone is safe and is becoming the sanitizer of choice to disinfect food products, clean equipment, sterilize water for bottling and disinfect high purity waters used to make pharmaceutical and health care products. It is made from the oxygen in the air and must be made and used on site because of its very short half life. Any unused ozone quickly reverts back to oxygen leaving no residues, making it an environmentally friendly sanitizer.
Ozone has been successfully applied for over 100 years as an oxidizer and disinfectant throughout many industries. It is exempted by the USEPA form any reporting, approved by the FDA as an antimicrobial agent for direct food contact and certified by the USDA as organic. It is approximately 3000 times faster than chlorine at killing bacteria, it inactivates virus and kills mold spores.
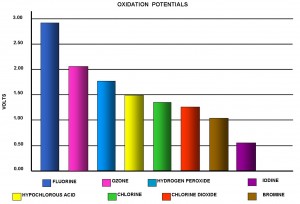
Ozone is the second strongest oxidizer next to fluorine and it is 150% stronger than chlorine. Applications requiring a strong and rapid sanitizer can benefit from the properties of ozone. The fact that oxygen is the only component of ozone assures the treated items will remain free of chemical residuals. The following graph compares the oxidation potential of many well known oxidizers.